Free OKR Templates
Are you working in the fast-paced world of pharmaceuticals? If so, you know the industry is full of exciting possibilities and significant challenges.
Whether you’re a researcher struggling to bridge the gap between promising discoveries and marketable drugs or a leader facing high clinical trial costs, you’re not alone.
This article is designed to be your one-stop shop for navigating the complexities of the pharmaceutical world. We’ll provide meaningful goals based on a proven framework for tackling these challenges.
17 OKR Templates For Pharmaceutical Companies
Challenge 1: Gap Between Discovery and Drug Development
1. Objective: Bridge the gap between promising scientific discoveries and successfully translating them into effective drugs
Owner: R&D Head & Translational Research Lead)
Due date: 3 months
- KR1: Increase the success rate of pre-clinical drug candidates advancing to clinical trials by 15%
- KR2: Establish a cross-functional group with R&D, clinical development, and regulatory affairs to address roadblocks in drug translation
- KR3: Implement a pilot program to test a new technology for early assessment of drug efficacy

Challenge 2: Data Overload and Analytics Capabilities
2. Objective: Enhance data analytics capabilities to extract meaningful insights and optimize drug development and commercialization
Owner: Chief Data Officer & R&D Head
Due date: 3 months
- KR1: Implement a new data management platform to integrate data from various R&D and clinical trial sources
- KR2: Develop and train internal data science teams on advanced analytics techniques for drug development decision-making
- KR3: Identify and implement two data-driven strategies for optimizing clinical trial design and resource allocation
Challenge 3: High Clinical Trial Costs
3. Objective: Reduce the average cost of conducting clinical trials by 10% within the next year.
Owner: Clinical Trial Management Head & Finance Director
Due date: 3 months
- KR1: Negotiate a 5% reduction in average vendor costs for clinical trial supplies and services
- KR2: Implement a centralized platform for clinical trial site selection and management to optimize resource allocation and reduce administrative costs
- KR3: Pilot a novel clinical trial design for one ongoing study to assess potential cost-effectiveness
Challenge 4: Diversity and Inclusion in R&D
4. Objective: Build a more diverse and inclusive R&D team to enhance innovation and address the needs of a broader patient population
Owner: HR Head & R&D Head
Due date: 3 months
- KR1: Increase the representation of women and minorities in R&D leadership positions by 20%
- KR2: Establish unconscious bias training programs for all R&D personnel
- KR3: Partner with a diversity-focused organization to develop and implement a patient-centric research design framework

Challenge 5: High-Pressure Environment, Burnout, and Employee Disengagement
5. Objective: Build a culture of well-being and employee engagement to reduce burnout and enhance productivity
Owner: HR Head & Department Heads
Due date: 3 months
- KR1: Reduce employee absenteeism due to stress-related illnesses by 10%
- KR2: Implement a company-wide employee wellness program with stress management and resilience training modules
- KR3: Conduct engagement surveys at the beginning and end of the quarter to measure progress and identify improvement areas

Challenge 6: Fierce Talent Competition, Talent Management, and Poor Retention
6. Objective: Attract, develop, and retain top scientific and research talent to maintain a competitive edge
Owner: HR Head & Department Heads
Due date: 3 months
- KR1: Develop and implement a competitive compensation and benefits package to attract top talent
- KR2: Launch a mentorship program pairing senior researchers with high-potential early-career scientists
- KR3: Reduce employee turnover within R&D and key scientific departments by 5%

Challenge 7: Outdated IT Systems and Data Integration
7. Objective: Modernize IT infrastructure and streamline data integration across departments to enhance efficiency and decision-making
Owner: CIO & IT Project Manager
Due date: 3 months
- KR1: Complete a comprehensive IT infrastructure assessment and develop a modernization roadmap
- KR2: Implement a pilot project to integrate data from two critical departments
- KR3: Develop and implement a training program for relevant personnel on new IT systems and data access protocols
Challenge 8: Shifting Regulatory and Approval Landscape
8. Objective: Maintain compliance with evolving regulations and expedite drug approval processes
Owner: Regulatory Affairs Head & R&D Head
Due date: 3 months
- KR1: Achieve a 100% success rate in internal regulatory audits
- KR2: Implement a dedicated regulatory intelligence team to monitor and analyze changes in regulatory requirements
- KR3: Reduce the average pre-submission meeting cycle time with regulatory agencies by 15%
VP of Quality (Chemical Manufacturing) Templates: Click here
Challenge 9: Balancing Profitability with Ethical Considerations and CSR
9. Objective: Balance the need for profitability with ethical drug pricing, responsible marketing practices, and commitment to corporate social responsibility
Owner: CEO & Marketing Director
Due date: 3 months
- KR1: Develop and implement a transparent pricing strategy that justifies drug costs and considers patient affordability
- KR2: Review and revise marketing materials to ensure accurate and ethical representation of drug benefits and potential risks
- KR3: Partner with a qualified organization to implement a CSR initiative focused on improving access to medication in underserved communities

Challenge 10: Rising Healthcare Costs and Pricing Pressures
10. Objective: Manage drug pricing effectively while demonstrating value to patients and payers
Owner: CEO & Market Access Head
Due date: 3 months
- KR1: Develop and implement value-based pricing models for two newly launched drugs
- KR2: Negotiate favorable reimbursement rates with key insurance companies for two high-impact drugs
- KR3: Conduct patient advocacy campaigns highlighting the value proposition and cost-effectiveness of your drugs compared to alternative treatments

Challenge 11: Patent Expiries and Competition from Generic Drugs
11. Objective: Mitigate the impact of patent expiries and ensure a robust pipeline of new drugs to maintain market share
Owner: R&D Head & Business Development Head
Due date: 3 months
- KR1: Accelerate the development of two new drugs in late-stage clinical trials by 10% to achieve market entry before competitor generics
KR2: Secure strategic partnerships or licensing agreements for two promising drug candidates in emerging therapeutic areas - KR3: Diversify revenue streams by exploring opportunities in non-branded generics or innovative drug delivery systems

Challenge 12: Supply Chain Disruptions and Shortages
12. Objective: Build a resilient supply chain to minimize disruptions and ensure uninterrupted access to essential medications
Owner: Supply Chain Head & Procurement Director
Due date: 3 months
- KR1: Identify and qualify two alternative suppliers for critical raw materials
- KR2: Implement a risk management strategy to assess and mitigate potential supply chain disruptions
- KR3: Increase finished product inventory levels for essential medications by 15% to improve buffer against potential shortages
Challenge 13: Counterfeit Drugs and Piracy
13. Objective: Combat counterfeit drugs and protect patient safety and brand reputation
Owner: Security Head & Legal Department Head
Due date: 3 months
- KR1: Implement a comprehensive anti-counterfeiting strategy including robust product authentication measures
- KR2: Collaborate with regulatory authorities and law enforcement agencies to investigate and prosecute counterfeit drug operations
- KR3: Develop and launch public awareness campaigns to educate about the dangers of counterfeit drugs
Challenge 14: Access to Affordable Medications in Developing Countries
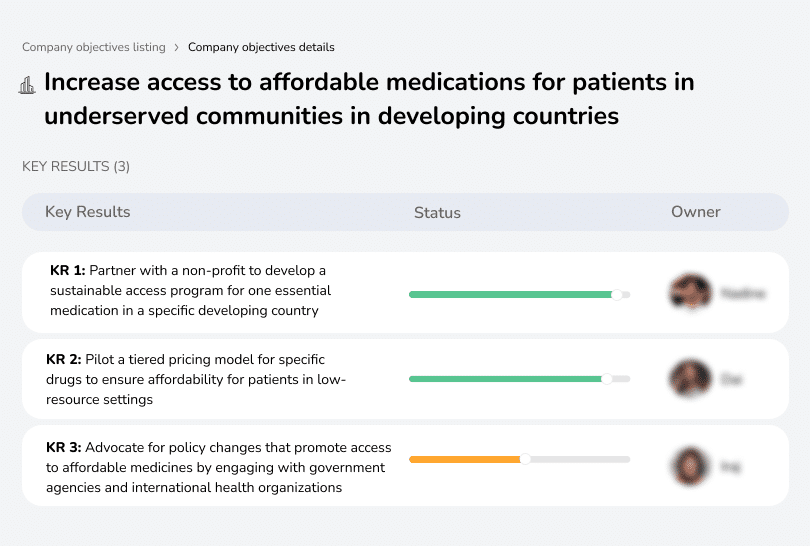
14. Objective: Increase access to affordable medications for patients in underserved communities in developing countries
Owner: Global Health Director & CSR Hea
Due date: 3 months
- KR1: Partner with a non-profit to develop a sustainable access program for one essential medication in a specific developing country
- KR2: Pilot a tiered pricing model for specific drugs to ensure affordability for patients in low-resource settings
- KR3: Advocate for policy changes that promote access to affordable medicines by engaging with government agencies and international health organizations
Challenge 15: Negative Public Perception and Reputation Management
15. Objective: Enhance the company’s public image by building trust and transparency with stakeholders
Owner: CEO & Communications Director
Due date: 3 months
- KR1: Increase positive media coverage about the company’s innovation, patient-centric approach, and social responsibility initiatives by 20%
- KR2: Launch a new patient advocacy platform to provide transparent information about drug development, clinical trials, and safety data
- KR3: Conduct a public perception survey to identify key areas for improvement and develop a targeted reputation management strategy
Challenge 16: Cybersecurity Threats and Data Protection
16. Objective: Protect sensitive patient information and intellectual property from cyberattacks
Owner: CIO & Security Head
Due date: 3 months
- KR1: Complete a comprehensive cybersecurity risk assessment and implement necessary security upgrades
- KR2: Train all employees on cybersecurity best practices and data protection protocols
- KR3: Conduct regular security audits and penetration testing to identify and address potential vulnerabilities within the IT infrastructure
Challenge 17: Geopolitical Issues and Trade Disputes
17. Objective: Mitigate the risks associated with geopolitical instability and trade disputes to ensure uninterrupted research and development activities
Owner: CEO & Government Affairs Director
Due date: 3 months
- KR1: Develop a comprehensive risk management strategy to identify and mitigate potential disruptions caused by geopolitical events
- KR2: Diversify research and development activities by establishing collaborations with research institutions in geographically diverse regions
- KR3: Advocate for policies that promote global cooperation and free trade through engagement with relevant international organizations